
In modern water treatment systems, the use of chemical coagulants and polymer flocculants has become widespread. However, many professionals in the industry still lack a comprehensive understanding of their working mechanisms. This article provides an in-depth explanation of how coagulants and flocculants function, helping wastewater engineers and treatment plant managers make more effective chemical selection and dosing decisions.
Coagulation and flocculation are two distinct but complementary processes in water treatment:
● Coagulation is the destabilization of colloids and suspended particles, primarily through neutralizing electrical charges.
● Flocculation is the aggregation of destabilized particles into larger particles (flocs).
Let’s explore the complex mechanisms behind both, which are crucial for effective treatment processes.
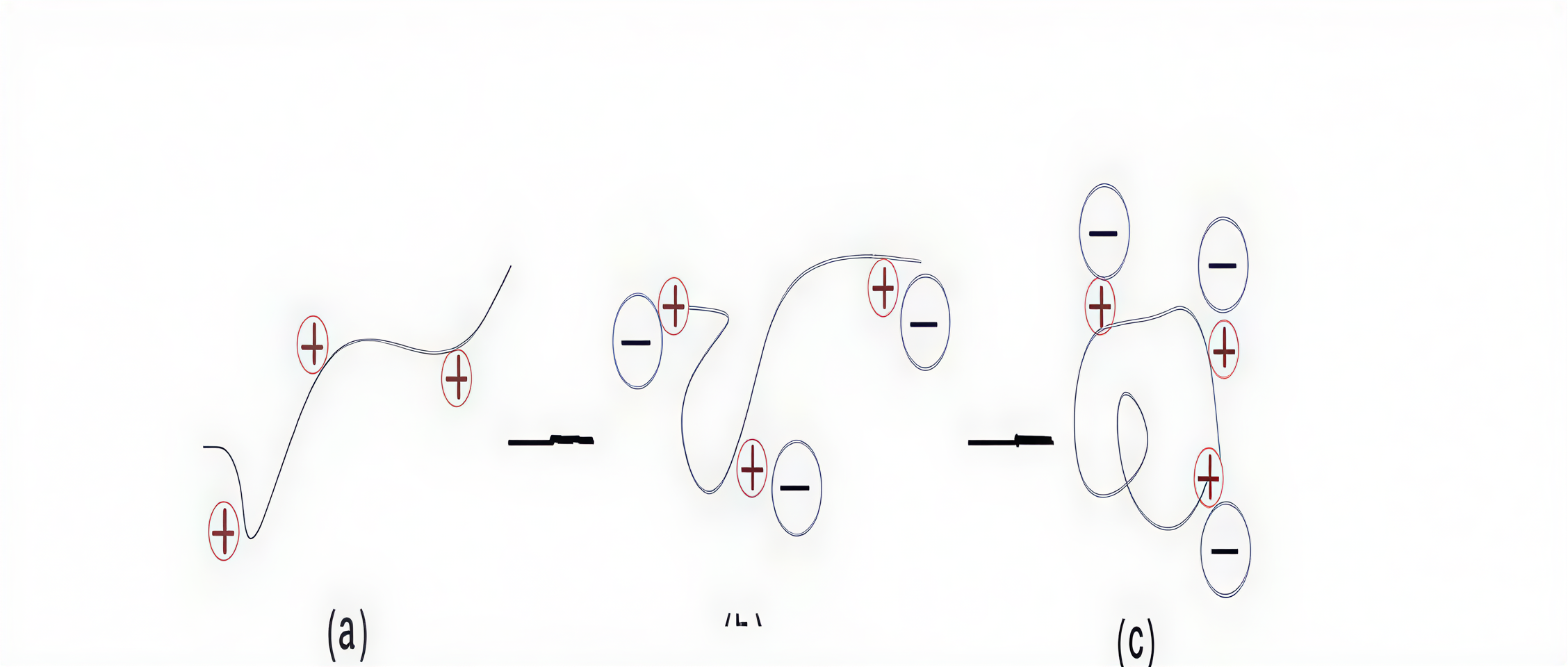
Coagulation involves the destabilization of colloidal particles, typically through the following mechanisms:
1.1 Double Layer CompressionAccording to the DLVO theory, adding multivalent cations compresses the electrical double layer around colloidal particles, reducing the zeta potential (ξ-potential). When the ξ-potential reaches a critical value or drops to zero (isoelectric point), electrostatic repulsion is eliminated, allowing particles to aggregate.
1.2 Adsorption and Charge NeutralizationColloids carry negative surface electrical charges that can be neutralized by oppositely charged ions or polymers, reducing repulsive forces and promoting particle aggregation. Coagulation is the process that targets this primary issue. Forces involved include:
● Electrostatic interactions
● Hydrogen bonding
● Coordination bonding
● Van der Waals forces
1.3 Adsorption BridgingHigh molecular weight polymer coagulants or inorganic coagulants can form "bridges" between particles, promoting floc formation. Bridging types include:
● Long-chain polymer bridging
● Short-distance bridging
Depending on the polymer charge and particle charge, bridging mechanisms vary in strength and effectiveness.
1.4 Sweep Flocculation (Enmeshment)Chemical coagulants like aluminum and iron salts (e.g., Ferric Chloride) hydrolyze in water to form hydroxide precipitates. These precipitates have a large surface area and can "sweep" or enmesh particles from the water mechanically as they settle.
Flocculation is the process of forming larger particles (flocs) from destabilized particles via:
2.1 Perikinetic Flocculation This is caused by Brownian motion, which leads to random particle collisions. It's most effective when particle sizes are small.
2.2 Orthokinetic FlocculationDriven by mechanical mixing or agitation, particles collide due to differences in velocity, forming larger flocs suitable for sedimentation or filtration.
In water treatment, "coagulation" often refers to the entire treatment processes from chemical coagulant dosing, mixing, particle destabilization, floc formation, and eventual sedimentation. It includes both the coagulation (destabilization) and flocculation (aggregation) phases.

4.1 Inorganic Coagulants Examples:
● Ferric ChlorideThese are water-soluble and typically added in liquid or powder form. They are effective but can be corrosive.
4.2 Organic Flocculants Mainly Polyacrylamide (PAM), which functions as a polymer coagulant:
● Anionic PAM is used for industrial wastewater flocculation, particularly in high-solids, low-charge wastewater.
● Cationic PAM is primarily used for sludge dewatering due to its strong positive charge affinity for negatively charged organic solids.
PAM must be dissolved before use and stored in a dry environment due to its hygroscopic nature.

Understanding the core mechanism ensures the efficiency of your facility's treatment processes.
(1) pH Value of WaterThe performance of chemical coagulants like aluminum salts depends heavily on pH. Optimal coagulation process occurs around pH 6.5–7.5. Outside this range, hydrolysis is incomplete, and efficiency drops. Organic polymer coagulants are less pH-sensitive.
(2) Water TemperatureLower temperatures reduce hydrolysis rates and hinder floc growth. In cold water, more coagulant may be required, but even then, flocs may be small and fragile. High temperatures can degrade organic polymer coagulants.
(3) Particle CharacteristicsParticle size and type (organic/inorganic) affect how well they coagulate.
● High turbidity usually improves performance, while extremely low turbidity may require auxiliary coagulants.
● Surfactants or negatively charged ions may hinder coagulation process.
(4) Flocculant TypeChoosing the right type of coagulant depends on water characteristics. In many cases, a combination of inorganic and organic flocculants works best.
(5) DosageThere is an optimal dosage range:
Inorganic chemical coagulants: 10–100 mg/L
● Organic polymer coagulants: 1–5 mg/L
● Overdosing can cause restabilization of particles, defeating the goal of the coagulation process.
(6) Dosing SequenceWhen using both inorganic and organic flocculants:
First add inorganic coagulant (e.g., PAC).
Then add the polymer flocculant (e.g., PAM) for bridging.
(7) Hydraulic ConditionsProper mixing is essential:
● Rapid mixing ensures even distribution.
● Gentle mixing allows larger particles (flocs) to grow without breaking apart.

To determine the optimal dosage for your treatment processes and compare the effectiveness of different chemical coagulants (e.g., Ferric Chloride vs. PAC) or polymer coagulants, a simple Jar Test should always be conducted in the laboratory.
The fundamental Jar Test steps are:
1. Sampling: Obtain sufficient amounts of raw wastewater.
2. Coagulation: Add varying doses of the primary coagulant, applying rapid mixing to ensure initial destabilization of electrical charges.
3. Flocculation: Add the polymer coagulant (flocculant), followed by slow, gentle mixing to promote the formation of larger particles.
4. Observation: Stop mixing and observe the floc settling rate and the clarity of the supernatant water.
Understanding the mechanisms behind coagulants and flocculants is essential for optimizing treatment performance and reducing chemical costs. By tailoring flocculant selection and dosing strategies to specific water conditions, industrial users can significantly improve efficiency and environmental compliance.
Need expert advice on choosing the right polymer coagulant or flocculant? Contact TAIRAN CHEMICAL for reliable solutions tailored to your wastewater treatment needs.